 Ozone Layer: A gaseous layer of the chemical ozone present in the atmosphere protecting the Earth from ultraviolet radiation. Ozone layer helps to produce the observed vertical structure of the atmosphere, and absorbs harmful ultraviolet radiation that would otherwise damage plant and animal life (also causing skin cancer) on the Earth’s surface. Hence Ozone is like an Anti-virus installed in our system which rescues our computer from virus threats.The ozone layer is thinnest near the equator and thickest at the poles. Since ozone formation depends on ultraviolet radiation from the Sun, the amount of ozone present in the atmosphere at any given time and place varies. Also, the lifetime of an ozone molecule in the stratosphere is between several months and several years, so the distribution of ozone is affected by the motion of the atmosphere; ozone molecules can be transported long distances before being destroyed.
Ozone Layer: A gaseous layer of the chemical ozone present in the atmosphere protecting the Earth from ultraviolet radiation. Ozone layer helps to produce the observed vertical structure of the atmosphere, and absorbs harmful ultraviolet radiation that would otherwise damage plant and animal life (also causing skin cancer) on the Earth’s surface. Hence Ozone is like an Anti-virus installed in our system which rescues our computer from virus threats.The ozone layer is thinnest near the equator and thickest at the poles. Since ozone formation depends on ultraviolet radiation from the Sun, the amount of ozone present in the atmosphere at any given time and place varies. Also, the lifetime of an ozone molecule in the stratosphere is between several months and several years, so the distribution of ozone is affected by the motion of the atmosphere; ozone molecules can be transported long distances before being destroyed.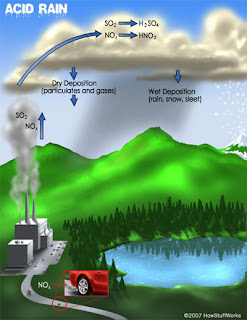 In contrast, ozone close to the Earth’s surface is a health hazard, as it is one of the major constituents of photochemical smog i.e., Low level ozone formed in the atmosphere from nitrogen oxides and organic gases emitted by cars and industrial sources, however, is a health hazard, and it may cause serious crop damage in some areas mainly through Acid Rains.
In contrast, ozone close to the Earth’s surface is a health hazard, as it is one of the major constituents of photochemical smog i.e., Low level ozone formed in the atmosphere from nitrogen oxides and organic gases emitted by cars and industrial sources, however, is a health hazard, and it may cause serious crop damage in some areas mainly through Acid Rains. Ozone is formed in the atmosphere when ultraviolet radiation from the Sun splits one oxygen molecule into two oxygen atoms (O2). The atomic oxygen then combines with another oxygen molecule to form ozone (O3). Most ozone found in the Earth’s atmosphere occurs in one layer in the stratosphere, between altitudes of around 20 to 50 km (12 to 30 mi).
Ozone is formed in the atmosphere when ultraviolet radiation from the Sun splits one oxygen molecule into two oxygen atoms (O2). The atomic oxygen then combines with another oxygen molecule to form ozone (O3). Most ozone found in the Earth’s atmosphere occurs in one layer in the stratosphere, between altitudes of around 20 to 50 km (12 to 30 mi).Now Ozone layer is in danger because of some chemical agents produced by human being. Now there is a need to save our ozone.
 The amount of chlorine in the atmosphere dramatically increased through the use and release of chemicals known as chlorofluorocarbons, or CFCs (compounds of fluorine). Which is used as a safe replacement for refrigerants, their chemical inertness also made them valuable in other areas of industry. Once released into the atmosphere, they were transported into the upper atmosphere where they were broken down by the much higher levels of ultraviolet. This is the only way in which CFCs released into the atmosphere can be destroyed. Almost all of the chlorine in the atmosphere is due to human activity. The most obvious danger from a reduction in the amount of ozone in the atmosphere is the increase in the amount of ultraviolet radiation reaching the surface, particularly the more dangerous UV-B.Compounds containing bromine, such as methyl bromide (mainly of natural origin) and the brominated CFCs (halons: used mainly as fire retardants), are also ozone-depleting chemicals.
The amount of chlorine in the atmosphere dramatically increased through the use and release of chemicals known as chlorofluorocarbons, or CFCs (compounds of fluorine). Which is used as a safe replacement for refrigerants, their chemical inertness also made them valuable in other areas of industry. Once released into the atmosphere, they were transported into the upper atmosphere where they were broken down by the much higher levels of ultraviolet. This is the only way in which CFCs released into the atmosphere can be destroyed. Almost all of the chlorine in the atmosphere is due to human activity. The most obvious danger from a reduction in the amount of ozone in the atmosphere is the increase in the amount of ultraviolet radiation reaching the surface, particularly the more dangerous UV-B.Compounds containing bromine, such as methyl bromide (mainly of natural origin) and the brominated CFCs (halons: used mainly as fire retardants), are also ozone-depleting chemicals.

The hydro-chlorofluorocarbons (HCFCs) were developed to replace CFCs. These gases can still damage ozone if they reach the stratosphere, but they are less likely to since their extra hydrogen atom allows them to be destroyed in the lower layers of the atmosphere.The gases that replaced both the CFCs and HCFCs are hydro-fluorocarbons (HFCs), which do not contain any chlorine atoms and so have no ozone depleting effect. So encourage and support is needed for HCFCs.
No comments:
Post a Comment