COUNTER
BIDVERTISER ADS
Saturday, May 24, 2008
Photovoltaics
Thursday, May 8, 2008
SOLAR ENERGY.

The strength of the solar energy available at any point on the Earth depends, in a complicated but predictable way, on the day of the year, the time of day, and the latitude of the collection point. Furthermore, the amount of solar energy that can be collected depends on the orientation of the collecting object.
What do you actually mean by CONSERVATION ??
what is sustainability?
Capable of being continued with minimal long-term effect on the environment.
Natural Resources:Natural resources are of two main types, renewable and non-renewable.
Renewable resources are inexhaustible or replaceable by new growth. These include wildlife and natural vegetation of all kinds. The soil itself can be considered a renewable resource, although severe damage is difficult to repair because of the slow rate of soil-forming processes. The natural drainage of waters from the watershed of a region can be maintained indefinitely by careful management of vegetation and soils, and the quality of water can be controlled through pollution control.
Non-renewable resources are those that cannot be replaced or that can be replaced only over extremely long periods of time. Such resources include the fossil fuels (coal, petroleum, and natural gas) and the metallic and other ores.
Need For Conservation!!!
1.Over Exploitaion of non-renewable resouces endagered the very existence of the human beings.
2.Deforestation: The large-scale removal of forest, prior to its replacement by other land uses. This is causing natural imbalances viz., Global warming, Acid rains, unseasonal rains, etc the most important life supporting gas Oxygen O2 content is reduced.
3.Soil Erosion: Erosion is broadly defined as removal of sediment, rock, and soil from the landscape, resulting in the formation of new landforms and the lowering of the land surface, a process known as denudation.Agents of Erosion: Water, Wind and Ice are the major agents of soli erosion.Fertility of soil is lost due to erosion, which affects plant growth.Here Soil Erosion and Deforestation are Interlinked.
4.Ozone Layer Destruction: Ozone Layer:A rerion of gaseous layer, which is found at about 40 km above sea level that shields the Earth from the Sun’s harmful ultraviolet rays.
5.Harmful Radiation: This is caused due to testing of nuclear weapons which is carried out by many supreme powers and developing countries of the world to show their superiority in destructive technology.
There are many such problems which cannot be listed in this blog. Hence, to save our environment and our planet it ultimately becomes every individuals duty to conserve environment.
Wednesday, May 7, 2008
Ozone(O3)
 Ozone Layer: A gaseous layer of the chemical ozone present in the atmosphere protecting the Earth from ultraviolet radiation. Ozone layer helps to produce the observed vertical structure of the atmosphere, and absorbs harmful ultraviolet radiation that would otherwise damage plant and animal life (also causing skin cancer) on the Earth’s surface. Hence Ozone is like an Anti-virus installed in our system which rescues our computer from virus threats.The ozone layer is thinnest near the equator and thickest at the poles. Since ozone formation depends on ultraviolet radiation from the Sun, the amount of ozone present in the atmosphere at any given time and place varies. Also, the lifetime of an ozone molecule in the stratosphere is between several months and several years, so the distribution of ozone is affected by the motion of the atmosphere; ozone molecules can be transported long distances before being destroyed.
Ozone Layer: A gaseous layer of the chemical ozone present in the atmosphere protecting the Earth from ultraviolet radiation. Ozone layer helps to produce the observed vertical structure of the atmosphere, and absorbs harmful ultraviolet radiation that would otherwise damage plant and animal life (also causing skin cancer) on the Earth’s surface. Hence Ozone is like an Anti-virus installed in our system which rescues our computer from virus threats.The ozone layer is thinnest near the equator and thickest at the poles. Since ozone formation depends on ultraviolet radiation from the Sun, the amount of ozone present in the atmosphere at any given time and place varies. Also, the lifetime of an ozone molecule in the stratosphere is between several months and several years, so the distribution of ozone is affected by the motion of the atmosphere; ozone molecules can be transported long distances before being destroyed.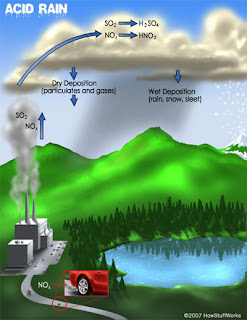 In contrast, ozone close to the Earth’s surface is a health hazard, as it is one of the major constituents of photochemical smog i.e., Low level ozone formed in the atmosphere from nitrogen oxides and organic gases emitted by cars and industrial sources, however, is a health hazard, and it may cause serious crop damage in some areas mainly through Acid Rains.
In contrast, ozone close to the Earth’s surface is a health hazard, as it is one of the major constituents of photochemical smog i.e., Low level ozone formed in the atmosphere from nitrogen oxides and organic gases emitted by cars and industrial sources, however, is a health hazard, and it may cause serious crop damage in some areas mainly through Acid Rains. Ozone is formed in the atmosphere when ultraviolet radiation from the Sun splits one oxygen molecule into two oxygen atoms (O2). The atomic oxygen then combines with another oxygen molecule to form ozone (O3). Most ozone found in the Earth’s atmosphere occurs in one layer in the stratosphere, between altitudes of around 20 to 50 km (12 to 30 mi).
Ozone is formed in the atmosphere when ultraviolet radiation from the Sun splits one oxygen molecule into two oxygen atoms (O2). The atomic oxygen then combines with another oxygen molecule to form ozone (O3). Most ozone found in the Earth’s atmosphere occurs in one layer in the stratosphere, between altitudes of around 20 to 50 km (12 to 30 mi).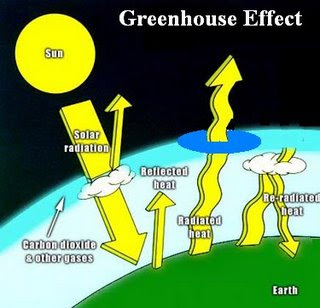 The amount of chlorine in the atmosphere dramatically increased through the use and release of chemicals known as chlorofluorocarbons, or CFCs (compounds of fluorine). Which is used as a safe replacement for refrigerants, their chemical inertness also made them valuable in other areas of industry. Once released into the atmosphere, they were transported into the upper atmosphere where they were broken down by the much higher levels of ultraviolet. This is the only way in which CFCs released into the atmosphere can be destroyed. Almost all of the chlorine in the atmosphere is due to human activity. The most obvious danger from a reduction in the amount of ozone in the atmosphere is the increase in the amount of ultraviolet radiation reaching the surface, particularly the more dangerous UV-B.Compounds containing bromine, such as methyl bromide (mainly of natural origin) and the brominated CFCs (halons: used mainly as fire retardants), are also ozone-depleting chemicals.
The amount of chlorine in the atmosphere dramatically increased through the use and release of chemicals known as chlorofluorocarbons, or CFCs (compounds of fluorine). Which is used as a safe replacement for refrigerants, their chemical inertness also made them valuable in other areas of industry. Once released into the atmosphere, they were transported into the upper atmosphere where they were broken down by the much higher levels of ultraviolet. This is the only way in which CFCs released into the atmosphere can be destroyed. Almost all of the chlorine in the atmosphere is due to human activity. The most obvious danger from a reduction in the amount of ozone in the atmosphere is the increase in the amount of ultraviolet radiation reaching the surface, particularly the more dangerous UV-B.Compounds containing bromine, such as methyl bromide (mainly of natural origin) and the brominated CFCs (halons: used mainly as fire retardants), are also ozone-depleting chemicals.

The hydro-chlorofluorocarbons (HCFCs) were developed to replace CFCs. These gases can still damage ozone if they reach the stratosphere, but they are less likely to since their extra hydrogen atom allows them to be destroyed in the lower layers of the atmosphere.The gases that replaced both the CFCs and HCFCs are hydro-fluorocarbons (HFCs), which do not contain any chlorine atoms and so have no ozone depleting effect. So encourage and support is needed for HCFCs.




